Using AI to control energy for indoor agriculture
30 September 2024
Published online 8 April 2010
Adding the new, investigational drug telaprevir to the existing therapeutic regimen for hepatitis C might improve the chances of recovery considerably in patients who had no success with treatment the first time. However, this drug does come with its share of troubling side effects.
Normally, hepatitis C is treated by a combination of peginterferon alpha-2a and ribavirin, which is an antiviral drug. This standard treatment is 1 year in duration, with a success rate of ~40%. For patients who do not respond, the only options are either to be put on sustaining medications or to repeat the same treatment regimen again. However, this rarely has successful results.
Researchers at the Duke Clinical Research Institute (DCRI) conducted a clinical trial on 453 multinational patients, divided into four test groups, who had previously failed to respond to hepatitis C treatment. Telaprevir was added to the standard regimen for 12 or 24 weeks.
More than 50% of the patients who previously had not responded to treatment with the standard regimen were virus-free 6 months after the study. "Efficacy here is three to four fold higher than what we get without this drug," John McHutchison, who is associate director for research at the DCRI and the lead author of the study, told Nature Middle East.
"This is a major development in the field and may represent the single most significant efficacy finding in the treatment of prior non-responders," he stated in a press release.
However, the group being treated with telaprevir did experience a significant side effect. About 50% of that group developed a rash, which ranged from mild to severe. A small percentage even had to stop treatment because the rash was too serious to continue.
"We are curing more people, but the side effects, particularly the rash and anaemia, led to more people abandoning the treatment, causing a higher rate of discontinuation," explained McHutchison.
He suggests, however, that having management programmes in place might help offset this problem. "Most of the rashes are mild or moderate and we can manage them with topical creams. In the more serious cases, telaprevir administration must be stopped."
The exact reason behind the rashes remains unknown. Currently there is no way to determine upfront who will develop the side effect. "Being able to identify that genetically would be very useful," added McHutchison.
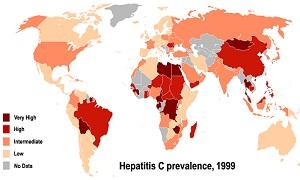
Hepatitis C is endemic to many countries in the Middle East and North Africa (MENA), with Egypt having the highest prevalence of the disease in the world. The research conducted at the DCRI focused on a certain variant of the hepatitis C virus (HCV) called genotype 1, which is the most prevalent type in the United States. However, in the MENA region, genotype 4 is the main source of infection.
According to McHutchison, one paper presented at a meeting last year showed that telaprevir had some activity against genotype 4, but it was a preliminary study. He explained that there are other direct antiviral drugs in the pipeline that are promising and might address several genotypes, "but these are still being developed and have not been the focus of this research."
"There are no antiviral drugs being developed locally to combat genotype 4 of HCV," said Alaa Ismail, who is director of the liver research unit in Ain Shams University, Egypt. "That is why all the drugs that we get from overseas must undergo clinical trials here again."
Previous research conducted by McHutchison showed that adding telaprevir to the standard regimen for hepatitis C can halve the treatment time. The next phase III study of telaprevir will test its efficacy in treating non-responders over shorter time spans.
The research was funded by Vertex Pharmaceuticals Incorporated, which also makes telaprevir.
doi:10.1038/nmiddleeast.2010.132
Stay connected: