Using AI to control energy for indoor agriculture
30 September 2024
Published online 19 August 2010
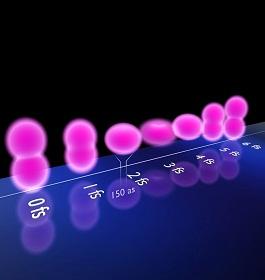
Chemical reactions between atoms occur at unimaginably short timescales. So it was a great step forward when researchers in the late 1980s were first able to take snapshots of these processes in action using ultra-short laser bursts1. Recently, physicists have extended this possibility into the realm of attoseconds (10-18 s).
Now an international team, including Abdallah Azzeer from King Saud University in Saudi Arabia, used a series of laser pulses each lasting just 150 attoseconds to record the shapes of krypton ions immediately after the outermost electron is knocked out of the atom by a high-intensity laser2.
The vacated shell starts to wobble with electrons constantly shifting between energy states in an attempt to settle down. Each of these quickly changing configurations absorbs different wavelengths of light. As the laser pulses contain a variety of frequencies, the physicists could interpret the ion's shape from those components of light absorbed by the ion. Their results show how the positive charge caused by the missing electron repeatedly alters between an elongated and a donut-shaped distribution.
According to theory, molecules prefer to break apart at points that draw electron holes. Insight into electron shifts, such as those of krypton, could lead to a better understanding of molecular interactions in currently incurable diseases, or help increase the speed of electronic data processing and transfer.
doi:10.1038/nmiddleeast.2010.189
Stay connected: