Using AI to control energy for indoor agriculture
30 September 2024
Published online 4 December 2011
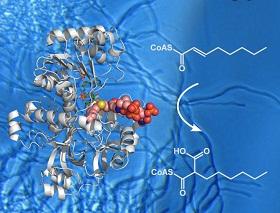
Polyketides are a large class of secondary metabolites that have become indispensible in the modern medicine. They are made by massive enzymes called polyketide synthases, which contain multiple catalytic domains, each of which can change the polyketide molecules in a different way.
Polyketide side chains, which branch off the main body of the molecules, exhibit a great deal of structural diversity that cannot be explained by the actions of polyketide sythases alone. However, recent research suggests that a class of enzymes called crotonyl-CoA carboxylase-reductase (CCRs) may be involved in the extension of polyketide.
Rolf Müller of Saarland University in Saarbrücken, Germany, and an international team of researchers including Schwan Rashid from the University of Koya, in Kurdistan, Iraq, isolated a CCR called hexylmalonyl-CoA synthase from the bacterial species Streptomyces sp. JS360.
The team are the first to crystalize the enzyme bound to its substrate, the small molecule whose addition to polyketides it catalyses. They then used X-ray diffraction to determine its structure at atomic-level detail, showing exactly how the enzyme binds its substrate. Their results are published in Nature Chemical Biology.
The study also identified two amino acid residues that are responsible for the specificity of the enzyme for a substrate.
By generating mutated versions of the enzyme, the researchers also show that these two residues are critical for binding carbon dioxide, which is required for the extension of polyketides.
"Our findings may lead to ways to deliberately alter and improve polyketides for clinical application," says Müller, "and we are now performing mechanistic and mutational studies of the enzyme."
doi:10.1038/nmiddleeast.2011.162
Stay connected: