Using AI to control energy for indoor agriculture
30 September 2024
Published online 14 December 2011
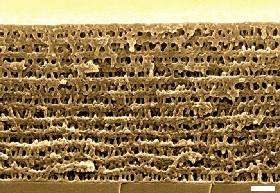
A study published in Nature Materials has demonstrated a cheap and simple way to construct nanoporous materials, which are used in filtration, catalysis and novel miniature electrodes, but expensive and time consuming to make.
A team of researchers, led by material scientist Easan Sivaniah at Cambridge University in the UK, and involving two researchers from Qatar University, Doha, used a technique called collective osmotic shock to rupture specific parts of a thin film of polystyrene filled with tiny spheres of polymethyl metacrylate (PMMA) to make a nanoporous material.
This thin film was exposed to ultraviolet light, breaking down the PMMA but leaving the polystyrene intact. Then, the material was dipped into acetic acid to remove the PMMA. This process swelled the spheres till the material fractured simultaneously under collective osmotic shock, leaving a porous polystyrene block.
Sivaniah and Shaheen Al-Muhtaseb at Qatar University developed the nanoporous material further to purify water by filtering out dyes of different sizes, controlling the pore size by adjusting the radius of the PMMA spheres. By replicating the film structure using metal oxide, the researchers generated a novel nanostructured electrode, which has applications in supercapacitors, photovoltaics and fuel cells.
"We demonstrated these applications" says Sivaniah, "we just need to optimize [the material] for various uses". The researchers also want to develop cheaper materials to make nanoporous films.
doi:10.1038/nmiddleeast.2011.167
Stay connected: