Using AI to control energy for indoor agriculture
30 September 2024
Published online 25 March 2013
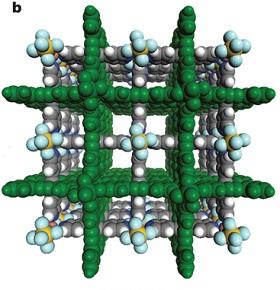
Researchers at King Abdullah University of Science and Technology (KAUST), Riyadh, and the University of South Florida in the United States have developed a cheap material that can trap carbon dioxide efficiently from gas mixtures.
Reporting their findings in Nature, the team demonstrated how an inexpensive, reusable metal-organic framework (MOF) can be used for carbon capture and separation with several industrial applications beckoning.
Currently, the energy costs associated with separating and purifying industrial commodities represents around 15% of global energy use. This material may lead to cost-effective tools for gas separation with lower energy consumption, especially as demand for these technologies increase.
They used 'crystal engineering', a design technique for synthesizing solid-state structure, to control the size of the pores in the metal-organic material (MOM). They then used negatively-charged ions to create favorable electrostatic interactions within the material. The end result was a porous inorganic-organic hybrid material with exceptional selectivity even in moisture.
The MOF material will be especially effective in capturing carbon dioxide from the waste gas pumped from power stations, fireplaces and boilers — the main source of CO2 emissions arising from human activities. Moreover, the material may be useful as a separation agent in the production of hydrogen and methane in power plants and fuel cells.
doi:10.1038/nmiddleeast.2013.35
Stay connected: