Using AI to control energy for indoor agriculture
30 September 2024
Published online 16 October 2014
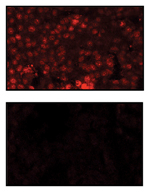
© KSR Sastry / Cell Death and Differentiation
Cancer stem cell (CSC) theory suggests that tumours contain a subpopulation of rare cells with stem-cell-like characteristics—capable of self-renewal and differentiation—that can drive tumour formation.
Most conventional cancer therapies target differentiated cancer cells but not CSCs, which would explain why chemotherapy does not always work.
Understanding the molecular mechanisms underlying the survival and differentiation capacity of CSCs might unlock potential targets.
The team in Qatar enriched and isolated CSCs from melanoma, breast and prostate cancers, and measured the levels of BAD and phosphorylated BAD in these cells.1
They found that CSCs have more phosphorylated BAD than differentiated cells. They mutated BAD in CSCs so that it can no longer be phosphorylated, and found that CSCs with this mutation had a decreased survival capacity, suggesting that BAD phosphorylation in CSCs mediates their ability to survive and self-renew.
Designing strategies to dephosphorylate BAD or drugs that target BAD phosphorylating enzymes may enable more potent cancer therapeutics in the future. BAD can also be used as an effective marker to follow tumour progression.
doi:10.1038/nmiddleeast.2014.247
Stay connected: