Using AI to control energy for indoor agriculture
30 September 2024
Published online 10 February 2015
New study shows why a certain condition can cause rapid uncontrolled mutations that can trigger cancers in children.
© Shlien, A. et al./Nature Genetics
Enlarge image
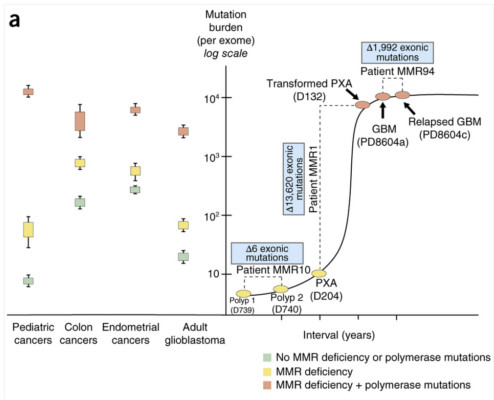
During replication, DNA undergoes various mutations, those that occur on both alleles of the same gene are called biallelic mutations. The cell normally recovers from the effect of these mutations through either polymerase proofreading or mismatch repair, two processes that will detect and replace any errors in the newly-synthesised strand.
However, in children with bMMRD, the rapid mutations thwarts their ability to repair any mismatch in DNA bases, triggering the growth of multiple brain tumours, lymphomas and gastrointestinal cancers. This makes bMMRD-linked growths, incidences of which are increasing in the Middle East, one of the most aggressive childhood cancers. Many children with bMMRD don’t reach adulthood.
A team of researchers led by Adam Shlien and Uri Tabori from the Hospital for Sick Children (SickKids) in Ontario, Canada, including Roula Farah from Saint George Hospital University Medical Center in Beirut, Lebanon, sequenced the cancer genomes of children with inherited bMMRD. They identified numerous point mutations in their tumour cells which outnumbered the mutation rates in all childhood and most other cancers.
When they analysed the tumour tissues, they found that the cancer cells had mutations in the genes that encode mismatch repair (MMR) proteins. They also had a secondary mutation in the gene that encodes polymerase enzyme which aids DNA replication.
The data suggests that the complete disruption of this DNA replication error repair system causes the cell to rapidly amass a huge number of point mutations over a very short period. These mutations continue to accumulate until a threshold is reached. At this point, biopsy analysis revealed that tumour cells couldn’t exceed the load of 20,000 mutations.
This high mutation load and threshold may be the cancer’s Achilles’ heel – pointing to possible therapeutic intervention; such as agents that may kill the cancer cells by pushing them over this mutational threshold.
“We can [also] use this information to develop novel diagnostic tools to find these children and monitor them and their families,” says Tabori, a lead author of the study and pediatric oncologist from the Hospital for Sick Children and the director of the International bMMRD Consortium. “We are currently developing a clinical trial with several centres in the Middle East with novel therapies based on these observations.”
The researchers hope their international collaboration will bring significant advancement in the management of this rare syndrome.
“Importantly, advanced genomic and genetic tools can improve our understanding of the mutational spectrum resulting in cancer in general, and offer hope for children with bMMRD” adds Shlien, who’s a geneticist and associate director of translational genetics at SickKids.
doi:10.1038/nmiddleeast.2015.25
Stay connected: