Using AI to control energy for indoor agriculture
30 September 2024
Published online 11 January 2015
© Panče Naumov/NYUAD
Enlarge image
But to be able to use them, it is important to first understand what happens on a molecular level when they bend, and how this is related to their macroscopic structure and behaviour.
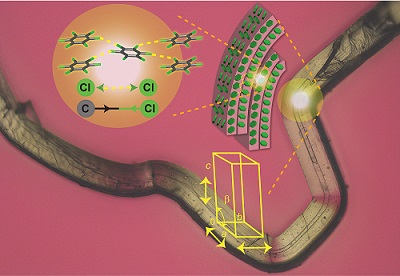
A team led by Panče Naumov of the New York University Abu Dhabi therefore looked at hexachlorobenzene (HCB) crystals1. These crystals have a peculiar property; upon impact on one face of the crystal, they undergo plastic deformation without breaking. However, impact on another face of the lattice can break the crystal, and the researchers set out to understand why.
They applied pressure on one face of the crystal and then inspected the lateral face. Electron microscopy revealed striations, suggesting that bending is related to molecular segregation into layers that can slide across each other. Melting point measurements showed that regions of different crystal density exist within the bent crystal, showing increased mosaicity.
They then used X-ray diffraction patterns to calculate the lattice structure along the bend and found that long-range order is maintained in the bent region. The increased mosaicity along the deformable face was due to breaking and reformation of the weaker Cl–Cl interactions.
But applying pressure to the opposing face, which has stronger interactions, would accumulate too much strain that would immediately break the crystals.
The researchers suggest this may not be unique to HCB, but could explain similar behaviour in other anisotropic molecular crystals, and shows how malleable these crystals can be.
doi:10.1038/nmiddleeast.2015.9
Stay connected: