Using AI to control energy for indoor agriculture
30 September 2024
Published online 18 April 2017
The RNA implicated in a congenital neurological condition that leads to developmental and learning delays.
© Mahmoud Elsaid / Tawfeq Ben Omran
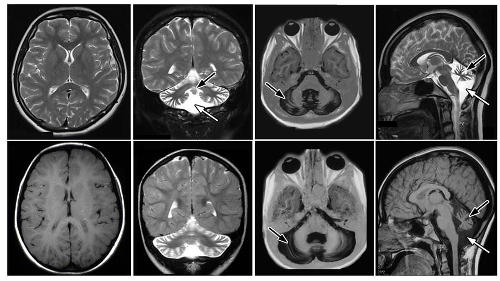
Congenital cerebellar ataxia results from poor development of the cerebellum, leading to an unsteady gait, developmental delays, poor muscle tone and coordination, and learning disabilities.
The cerebellum, located in the back of the skull, is important for muscle coordination.
A team of researchers led by Alice Abdel Aleem, a neurogeneticist at Weill Cornell Medicine–Qatar, analysed DNA, RNA and proteins extracted from 18 Qataris belonging to two related families, of whom six out of 14 children have the condition. The children were born into consanguineous marriages.
Comparisons of the genomes of 11 members belonging to one family identified a mutation in a gene, called RNU12, as the cause behind cerebellar ataxia. RNU12 is a non-protein coding gene involved in editing more than 850 other genes.
Most disease-causing mutations identified to date have been found in the proportionately small protein-coding part of the genome. But it is becoming increasingly clear that this part of the genome does not account solely for inherited human diseases.
“The non-coding regions that regulate how the genetic blueprint is read by our cells pose a new frontier of genetic investigation that has been challenging to explore,” says Abdel Aleem. But, sequencing the whole genome to identify mutations and then providing solid evidence they cause disease requires a tremendous amount of work, she says.
The team’s analyses revealed that the mutation in RNU12 was affecting its RNA’s normal ability to ‘splice’, or extract, nucleotide sequences, called minor introns, from genes known to be associated with cerebellar neurodegeneration.
This is the first report of a mutation in this particular kind of splicing RNA causing congenital cerebellar ataxia in humans and is only the second example of it being found to cause human disease.
“The study will open a new window for screening for cerebellar ataxia. It also shows geneticists how important it is to conduct research on all parts of the genome — both protein-coding and non-coding — when looking for causes of disease,” says Abdel Aleem.
Since consanguineous marriages are common in Qatar, people who are found to carry the mutation, which is inherited in a recessive manner, could avoid marrying other carriers to protect their children from inheriting the disease. Married carriers could also undergo prenatal genetic implantation, which involves using an ovum and sperm devoid of the mutation.
“We hope, in the near future, to discover drugs that can lessen the severity of the manifestations and the progression of the disease,” adds Abdel Aleem.
The team plans to develop an animal model that is engineered to carry the mutation in collaboration with researchers at Weill Cornell Medical College in New York. This will help them further understand which genes are directly affected by the RNU12 mutation and to what extent it impacts the cerebellum. “This is a crucial step toward discovering therapeutics for the condition,” says Abdel Aleem.
doi:10.1038/nmiddleeast.2017.68
Stay connected: