Using AI to control energy for indoor agriculture
30 September 2024
Published online 24 September 2018
Controlling the formation of gold–platinum alloy nanoparticles unleashes the catalytic power of single platinum atoms.
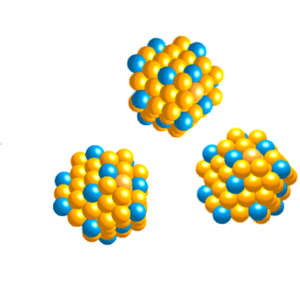
Nature Materials 2018
“Our work will open up a new avenue for designing high-performance catalysts using the minimum amount of platinum metal,” says nanoscientist Peng Zhang of Dalhousie University in Halifax, Canada. Zhang worked on developing the innovation with colleagues in the USA, UK, China and at King Saud University in Saudi Arabia.
Many platinum catalysts suffer from carbon monoxide ‘poisoning’, in which carbon monoxide molecules bind to the catalyst and eventually deactivate it. The new procedure avoids this due to its alloyed structure, in addition to yielding a catalytic performance that is around 100 times greater than existing commercial platinum catalysts.
The catalysts assemble in chemical mixtures called colloids, which have many of the properties of solutions but with tiny solid-state components suspended within the liquid phase. This method of formation allows control over the way that gold and platinum atoms combine, and generates a very high surface packing density of isolated platinum atoms.
The unique power of the catalysts has already been shown to greatly improve formic acid oxidation, a reaction with developing applications in fuel cell technology.
“We hope our method will also prove more generally useful, especially in many other catalytic processes where carbon monoxide poisoning is a problem,” says Zhang.
The researchers are now exploring ways to replace the gold in the nanoparticles with less expensive metals to move toward cost-effective commercial applications.
doi:10.1038/nmiddleeast.2018.118
Zhang, P. et al. Golden single-atomic-site platinum electrocatalysts. Nat. Mater. http://dx.doi.org/10.1038/s41563-018-0167-5 (2018).
Stay connected: