Using AI to control energy for indoor agriculture
30 September 2024
Published online 28 February 2019
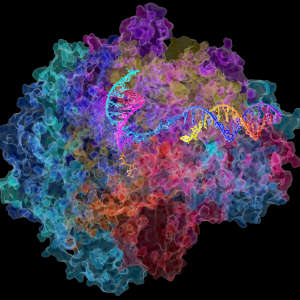
RNA polymerase II (Pol II) removes RNA errors with the help of an oxygen atom in the target RNA nucleotide.
Science History Images / Alamy Stock Photo
RNA polymerase II plays a critical dual role during the genome transcription process, which leads to the formation of the proteins responsible for carrying out cellular functions. First, it transcribes DNA into RNA. Then, it removes wrongly transcribed nucleotides to protect the cell from genetic errors that can result in cell stress or even death.
However, the precise molecular mechanism by which Pol II executes the removal had not been fully known.
The research team, including a scientist from King Abdullah University of Science and Technology (KAUST) in Saudi Arabia, used computational methods to simulate the chemical reaction mediated by Pol II to excise its target nucleotides, which are composed of a nitrogenous base and a sugar molecule bound to a phosphate group.
This approach suggested that Pol II utilises an oxygen atom in the target nucleotide’s phosphate group to excise the error rather than being dependent on a specific residue in the Pol II enzyme itself, as was initially thought. This theoretical prediction was further validated experimentally, supporting the novel finding.
“Our proposed mechanism suggests that the Pol II compartmentalises its backtracking action, where the enzyme pauses the transcription to start the RNA proofreading, from the cleavage reaction that cuts the wrongly transcribed nucleotides,” says Xuhui Huang, a chemist from The Hong Kong University of Science and Technology (HKUST).
“While the backtracking requires Pol II residues, it turns out that the cleavage reaction remarkably does not,” adds Huang.
doi:10.1038/nmiddleeast.2019.29
Tse, C. K. et al. Intrinsic cleavage of RNA polymerase II adopts a nucleobase-independent mechanism assisted by transcript phosphate. Nat. Catal. https://doi.org/10.1038/s41929-019-0227-5 (2019).
Stay connected: