Using AI to control energy for indoor agriculture
30 September 2024
Published online 26 June 2019
A deep look into ‘junk’ DNA yields insights into liver regeneration.
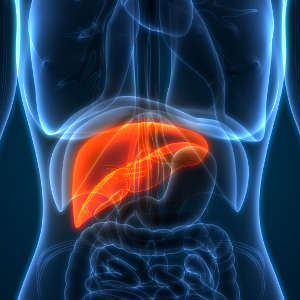
magicmine / Alamy Stock Photo
The liver is the only mammalian organ capable of regeneration, even after injury or extensive surgery. A wealth of data indicates that epigenetic mechanisms, which influence how genes are activated or silenced without altering the DNA sequence, play a key role in this remarkable recovery process.
Now, researchers exploring the effects of turning off an epigenetic regulator in mice involved in cell proliferation, called UHRF1, have made a surprising discovery. They expected UHRF1 loss to block liver cell proliferation. Instead, they found that mice lacking UHRF1 showed enhanced regeneration following partial liver removal.
“I immediately thought of all the technical reasons why the mice were healthy and had what appeared to be completely normal liver physiology,” says developmental biologist, Kirsten Sadler Edepli of New York University Abu Dhabi (NYUAD). After numerous tests to be sure the results were not just a one-off, “it turned out that the fascinating world of transposons provided the answer,” she says.
Transposons — short sequences of DNA found in what is often called the ‘junk’ DNA region — are thought to be the remains of ancient viruses that can cause genomic instability if activated. Many studies using zebrafish and mice have shown that UHRF1 is essential for DNA methylation, a process involving the addition of methyl groups to DNA, which can crucially silence these potentially harmful transposons.
Sadler Edepli and her team found that reduced DNA methylation in mice lacking UHRF1 intriguingly did not ‘wake up’ transposons. To account for this, the team suggests an inbuilt protection mechanism is at work to ensure the transposons remain dormant.
They suggest that enhanced liver regeneration happens when a gene-silencing epigenetic mark called H3K27me3 is repositioned from gene promoters, which initiate gene replication, to transposons. This has a dual effect of lifting the suppression of pro-regenerative genes and silencing transposons.
The newly uncovered mechanism that appears to manage the threat of transposons could be therapeutically relevant. More research will be needed before translating to the clinic, however. “Until we understand how cells and whole organisms compensate for epigenetic changes, I think cautious optimism is the best approach,” says Sadler Edepli.
doi:10.1038/nmiddleeast.2019.92
Wang, S. et al. Epigenetic compensation promotes liver regeneration. Dev. Cell 50, 1–14 (2019).
Stay connected: