Using AI to control energy for indoor agriculture
30 September 2024
Published online 5 February 2020
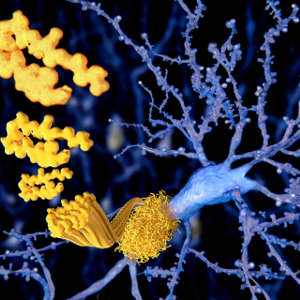
Study reveals a promising route for inhibiting the aggregation of harmful proteins associated with Alzheimer’s and other amyloid diseases.
Science Photo Library / Alamy Stock Photo
Now, an international group of scientists has proposed a new and effective way for targeting Aβ using short, engineered amino acid sequences called cell-penetrating peptides (CPPs)1 .
“CPPs are a class of peptides that enter cells with high efficiency and low toxicity,” explains Mazin Magzoub of New York University Abu Dhabi. “Importantly, CPPs may also readily cross the blood–brain barrier. They combine many attractive properties intrinsic to peptides, such as high target specificity and selectivity, biocompatibility and biodegradability, as well as ease and low cost of production, with potent therapeutic effects.”
The research team took inspiration from precision-engineered CPPs that some of its members had previously designed2, 3 as a treatment strategy for prion diseases, a group of neurodegenerative disorders caused by abnormal clumping of mis-folded proteins in the brain. These CPPs, comprised of a water-repelling sequence of amino acids fused to a positively charged, amyloid-derived sequence, were successfully shown to inhibit the conversion of normal cellular prion proteins into their disease-associated form.
“Here we extended this approach towards Alzheimer’s disease by demonstrating that these CPPs effectively inhibit Aβ aggregation and their associated neurotoxicity,” says Magzoub.
The team showed that the CPPs inhibit Aβ clumping by selectively binding to Aβ thanks to the targeted nature of their designed sequence. The inhibitory effect is thought to be due to the higher stability of Aβ–CPP interactions compared to Aβ’s self-interaction.
He points out that Aβ is inherently prone to aggregation, making it notoriously difficult to handle. One of the challenges the team faced was determining the experimental conditions that would allow them to study Aβ, particularly when using techniques that require high peptide concentrations, such as nuclear magnetic resonance spectroscopy, which was needed to determine the binding interactions between the CPPs and Aβ. The international collaboration, which included biophysicists and biochemists from institutes in the United Arab Emirates, USA, Sweden and Estonia, was critical to overcoming this challenge, he says.
Due to the effectiveness of their strategy in two different amyloid systems, Magzoub suggests that the CPPs could function as general amyloid inhibitors, meaning that their findings could have applications in the treatment of many amyloid-related diseases.
One of the team’s next goals is to investigate whether their CPP-based strategy is effective against other amyloid proteins such as α-synuclein, implicated in Parkinson’s disease, and islet amyloid polypeptide, associated with type II diabetes.
doi:10.1038/nmiddleeast.2020.20
Stay connected: