Using AI to control energy for indoor agriculture
30 September 2024
Published online 17 August 2021
A versatile new type of polymer has been built using 'click chemistry'.
Han Zuilhof 2021
Enlarge image
Sharpless coined the term click chemistry to describe reactions that link molecules, like clicking together construction toys. This mimics how nature builds polymers, such as proteins, DNA and RNA.
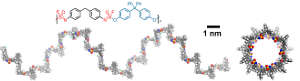
The new polymers are built using a click reaction called Sulfur(vi) fluoride exchange (SuFEx) to combine alternating molecules of bis(iminosulfur oxydifluorides) and bis(aryl silyl ethers). These become held together by a sulfur atom link.
Sharpless says he expected a complex mixture would arise from the reaction, but surprisingly it produced quite pure single structures. The polymer molecules can be modified by further SuFEx reactions, attaching new side groups to offer a wide range of chemical possibilities.
Another surprise came when electron microscopy analysis, performed at KAU, revealed a regular helical structure, somewhat similar to those of DNA and parts of proteins.
“We don’t know why we have these beautiful helices,” says researcher Han Zuilhof of Wageningen University in the Netherlands, who has an affiliation with KAU. That is something he hopes to investigate further. He would also like to find easier ways to make thionyl tetrafluoride, the basic starting material of the monomers. “This would allow many more people to access [the chemistry],” he says
Zuilhof emphasizes that this work is at present fundamental chemical research. The next priority is to learn more about the chemistry controlling the structure and reactivity of the polymers. But he does suggest some possibilities for eventual applications.
Attaching other chemical groups at regular intervals might make new catalysts for reactions useful in research, industry or medicine. Other possibilities include attaching binding sites for microbes to create anti-microbial therapies or diagnostic tests.
doi:10.1038/nmiddleeast.2021.70
Li, S. et al. SuFExable polymers with helical structures derived from thionyl tetrafluoride. Nat. Chem. https://doi.org/10.1038/s41557-021-00726-x (2021).
Stay connected: