Special Issue: Water Narratives
24 December 2025
Published online 25 October 2021
A deep dive into an unusual case of severe diabetes reveals a gene involved with pancreatic development that could play a role in disease for a larger number of patients.
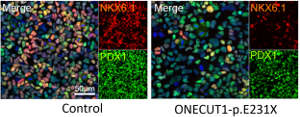
Sandra Heller and Alexander Kleger / Reprinted from extended data (Fig 2) of Philippi, A. et al. Mutations and variants of ONECUT1 in diabetes. Nat. Med. https://doi.org/10.1038/s41591-021-01502-7 (2021).
Enlarge image
The study began when co-lead author, Cécile Julier of the Université de Paris, and collaborator Marc Nicolino, at the Hospices Civils de Lyon, learned of a family in which a child had died of severe neonatal diabetes just months after birth. An initial investigation revealed that this disease was caused by two mutated copies of a gene called ONECUT1.
Julier and colleagues embarked on a genetic and medical investigation of the child’s extended family. “We showed that individuals who carried one mutated copy of ONECUT1 had increased risk of adult-onset diabetes,” says Julier. They then cast a wider net, and found additional cases linked to this gene, including a patient in Lebanon, identified by co-author Pierre Zalloua of the University of Balamand. Their analysis revealed a host of different ONECUT1 mutations, all linked to risk of diabetes with varying degrees of severity.
To get to the bottom of this gene’s function, co-lead author, Alexander Kleger, of Ulm University, used a human stem cell model to determine how disruptions of ONECUT1 function affect pancreatic development and function. “These meticulous laboratory investigations showed that formation of pancreatic progenitor cells was substantially suppressed by the newly discovered ONECUT1 mutations,” says Julier. Indeed, the initial index patient exhibited a severely underdeveloped pancreas.
Toni Pollin, who studies genetics of diabetes at the University of Maryland, says the group’s deep dive into the biology of ONECUT1, which had previously been linked to diabetes but never fully characterized, “shows the real importance of methods that rely more on thinking about the phenotype and what you're observing clinically, and focusing on specific genes. It’s a beautiful example of taking what was found in these human cases and using it to move the biological understanding forward.”
Fully monogenic cases of ONECUT1-linked diabetes may be rare, but this study suggests that single mutated copies of this gene may be at play in a larger number of patients. “These patients may benefit from more personalized diabetes treatment,” says Julier, although additional research will be needed to clarify the mechanisms by which different mutations contribute to this condition.
doi:10.1038/nmiddleeast.2021.85
Philippi, A. et al. Mutations and variants of ONECUT1 in diabetes. Nat. Med. https://doi.org/10.1038/s41591-021-01502-7 (2021).
Stay connected: