Using AI to control energy for indoor agriculture
30 September 2024
Published online 4 July 2022
Asymmetric pores built into a metal-organic framework offer a more cost-effective way to purify methane and other gases.
Nature 606 (2022)
Enlarge image
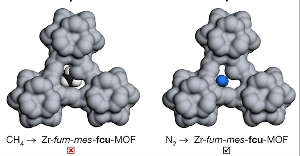
Mohamed Eddaoudi’s team at King Abdullah University of Science and Technology (KAUST) has developed a metal-organic framework (MOF) membrane with the potential to reduce the cost of nitrogen removal during methane purification by about 66%. The researchers predict that the cost of removing nitrogen and carbon dioxide simultaneously could be reduced by as much as 73%. Advances on this scale could greatly assist the global effort to use methane as a fuel and chemical feedstock instead of fossil fuels.
MOFs contain metal ions or metallic clusters held together by organic molecules called linkers. These versatile hybrid materials have reticular structures containing pores whose geometry and chemical properties can be fine-tuned by varying the metal and linker groups. The KAUST team developed a zirconium-based MOF with asymmetric pores that block the passage of tetrahedrally-shaped methane molecules while allowing linear-shaped nitrogen to pass through for subsequent removal. The unusual but crucial asymmetry of the pores was achieved by replacing some of the linkers, built from the organic chemical fumarate, with the modified version called 2-methyl fumarate. This converts originally symmetrical trefoil-shaped pores that will allow methane to pass into the asymmetric structures that methane cannot penetrate.
The researchers report that membranes built from their MOF achieved record-breaking selectivity when separating nitrogen from methane. The membranes could operate at up to 50 times atmospheric pressure, as required for practical and efficient methane purifying applications.
“This is a testament to the uniqueness of MOF chemistry and the power of reticular chemistry,” says Eddaoudi.
Professor of chemistry, David Fairen-Jimenez, at the University of Cambridge, UK, who was not involved in the research, says “Nitrogen gas is probably the most challenging to remove [from methane]. This work brings a simple and exciting solution. Membrane separation is an exciting technology where the molecular control of MOF crystals brings many potential advantages.”
The team also demonstrated effective separation of carbon dioxide from methane and believe their designer MOFs have the potential to separate other industrially relevant gas pairs. These include separating hydrogen from methane or nitrogen, and carbon dioxide from nitrogen.
In general, Eddaoudi describes the ability to use MOFs to separate similarly sized molecules based on their shape as “a game changer.” He predicts great success in developing MOFs to replace the highly energy-intensive distillation-based processes currently used to separate many gas mixtures in the chemical industry.
doi:10.1038/nmiddleeast.2022.36
Zhou, S. et al. Asymmetric pore windows in MOF membranes for natural gas valorization. Nature 606, 706-715 (2022).
Stay connected: