Special Issue: Water Narratives
24 December 2025
Published online 31 January 2023
Analysis of a mutated gene underlying a rare neurological disorder offers a view into the mechanisms guiding early brain development.
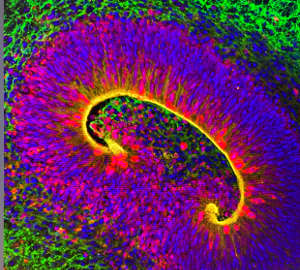
Joseph Gleeson, University of California at San Diego
There are a number of hereditary conditions in which this folding process—known as gyrification—gets derailed. One such condition, polymicrogyria (PMG), which leads to excessive formation of abnormally small folds, offered an essential starting point for both studies. “This all started with a patient of mine with profound global developmental delay and cortical malformation,” explains Fowzan Alkuraya, of the King Faisal Specialist Hospital and Research Center in Riyadh, Saudi Arabia. His team tentatively linked this patient’s PMG to a mutation in a gene known as TMEM161B, and joined forces with a larger effort led by Christopher Walsh at Boston Children’s Hospital to analyse this gene’s role in brain development. In parallel, researchers led by Joseph Gleeson, of the University of California at San Diego, learned of four families with hereditary neurological problems linked to TMEM161B, thanks to collaborator Maha Zaki, at the National Research Centre in Cairo, Egypt.
Little was known about this gene. On one hand, it bears no meaningful resemblance to other genes with known roles in brain development. Nevertheless, TMEM161B is represented even in some of our most distant evolutionary relatives—including plants and amoebae—indicating that it clearly has an important job. Both teams therefore set out to unravel TMEM161B’s function.
Walsh and colleagues found strong evidence from mouse and human tissues that this gene is specifically active in outer radial glial cells, which coordinate neuronal organization and contribute to the gyrification process. Mice lacking this gene died shortly after birth with massive defects in brain development, including improper separation of the brain’s two hemispheres. The researchers confirmed TMEM161B’s role in fold formation by disrupting the gene’s function in the developing brains of embryonic ferrets—a species whose brain undergoes gyrification, unlike the smooth brains of rodents. Walsh’s team also found evidence that the TMEM161B protein influences gyrification through its interaction with the Sonic Hedgehog signalling pathway, a critical regulator of brain development.
Gleeson’s group used a more human-centric experimental model, deriving three-dimensional ‘organoids’ that recreate features of the human brain from stem cells donated by people with PMG and their families. Organoids with TMEM161B mutations were disorganized compared to those from cells without such mutations and, like the Walsh team, the researchers linked these perturbations to abnormalities in the radial glial cell population. But they also observed distinctive cellular defects in their organoid and mouse models, primarily affecting the glial cell fibres that provide essential infrastructure for neuronal organization. “These fibres provide guidance for immature neurons to shimmy up to their correct spot in the grey matter of the brain,” says Gleeson. He adds that loss of TMEM161B disrupts the fibres, stranding these neurons in the wrong place, prompting the formation of extra brain folds.
Victor Borrell, who studies cortical development at the Universidad Miguel Hernández in Spain, is excited about the complementary results of the two studies. “These findings are important for the genetic diagnosis of brain malformations in patients, and to further our understanding of the normal cellular and molecular mechanisms of cortex folding, which remains a major enigma,” he says. But he also notes that the animal data do not fully capture the manifestations of PMG seen in humans, and that better models will be needed to fully understand how the gyrification process plays out in humans.
doi:10.1038/nmiddleeast.2023.10
Stay connected: