Using AI to control energy for indoor agriculture
30 September 2024
Published online 22 March 2023
A chance observation due to dust contamination has uncovered a surprising new process of fluid transport when a crystal surface interacts with water.
Nature Chem (2023)
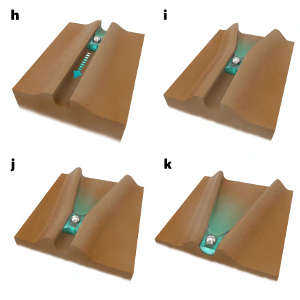
A group led by chemist, Panče Naumov, worked with crystals of hexachlorobenzene to explore its behaviour, particularly its “mechanical compliance”, which might make it useful in producing soft and flexible structures.
Researcher, Patrick Commins, explains the novel discovery came as a complete surprise after some dust fell on a sample. “We were all shocked when we saw the dust spontaneously start to move on the crystal,” he says.
Marieh Al-Handawi, who also worked on the study, adds that learning how the movement was driven by water droplets condensing on the crystal came as another surprise. “It results in the formation of channels that allow water to flow down them just like a river,” she says.
The channels form and then autonomously direct the flow of water due to a subtle and previously unrecognized phenomenon. Water condensing on to the surface initiates the conversion of some of the solid hexachlorobenzene directly into vapour – a process called sublimation. This forms tiny widening channels that direct the water in one direction within each channel. The movement of dust particles propelled by the water suggested it could be used to move other solids, in addition to fluid solutions. The team confirmed this using silver nanoparticles. They also found that the speed of transport along the channels could be varied depending on temperature and humidity.
There are significant challenges to address before this phenomenon could be put to practical use. It does lead to loss of the crystal, albeit at quite a slow rate. Al-Handawi says that about 90% of the crystal mass would be lost in around 271 days at room temperature. This would be viable only in single-use or relatively short-term applications.
Also, the direction of water transport differs in neighbouring channels. Obtaining uni-directional transport throughout the crystal will be challenging, but could greatly expand the potential applications. The team has already made some progress towards that goal.
Chemist, Yongmei Zheng, at Beihang University in China, who was not involved in the work, says that the advance has the potential to inspire a variety of new uses for the surface transfer of water without requiring external energy.
doi:10.1038/nmiddleeast.2023.21
Commins, P. et al. Autonomous and directional flow of water and transport of particles across a subliming dynamic crystal surface. Nat. Chem https://doi.org/10.1038/s41557-023-01158-5 (2023).
Stay connected: