From Architecture to AI-Powered Building Diagnostics: Tarek Rakha’s start-up story
10 June 2025
Published online 12 April 2023
A lab-scale demonstration reveals a surprisingly simple way to make ammonia from nitrogen and water.
Richard N. Zare
Enlarge image
Ammonia is used to make fertilisers that provide the essential nitrogen needed by plants in a form they can absorb, since they cannot directly take up and use the nitrogen that comprises 78% of the air by volume. It is also a major feedstock for making a wide variety of other industrial chemicals.
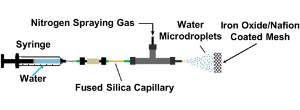
Nitrogen molecules are extremely stable, with their two nitrogen atoms held together by three strong covalent bonds. This stability is overcome in the Haber-Bosch process by combining nitrogen with hydrogen at around 400oC and more than 150 atmospheres of pressure with the assistance of an iron-based catalyst. The new method works when water droplets at room temperature are mixed with nitrogen and sprayed through a graphite mesh coated with iron oxide (magnetite) and a polymer called Nafion (a sulfonated tetrafluoroethylene-based fluoropolymer copolymer).
“I was thrilled to discover that a simple room-temperature and atmospheric-pressure process could turn [nitrogen] into ammonia,” says chemist, Richard Zare of the Stanford team. The work reveals that the simple trick of applying water in the form of micro-droplets sufficiently increases its chemical reactivity for the reaction to proceed on the surface of the catalyst mesh.
At present, the reaction has been achieved as only a very small-scale laboratory demonstration, with the creation of ammonia detected using a sophisticated instrument called a mass spectrometer. The team is now investigating whether the process could feasibly be scaled up and the ammonia concentrated sufficiently for commercial application.
Chanbasha Basheer, a chemist at King Fahd University of Petroleum and Minerals, who was also involved in the study, says that ammonia production from air, using water as a source of hydrogen, could become more sustainable and efficient with further improvements in catalysts, energy efficiency and scale-up.
doi:10.1038/nmiddleeast.2023.29
Song, X. et al. Making ammonia from nitrogen and water microdroplets. PNAS 120, e2301206120 (2023).
Stay connected: