Using AI to control energy for indoor agriculture
30 September 2024
Published online 7 July 2014
Researchers uncover how some crystals can jump large distances when exposed to ultraviolet light, which could pave the way to actuators driven by light instead of electricity.
© Ahmad Husain
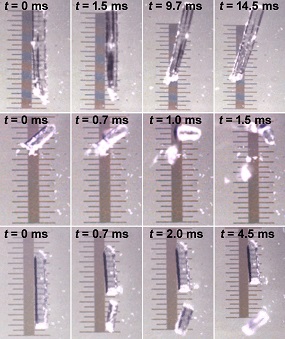
While working with single crystals of zinc metal coordination complexes, Raghavender Medishetty at the National University of Singapore (NUS) found that crystals jump to heights several times their sizes when exposed to UV light in a similar vein as the popping of corn kernels at high temperatures – a phenomenon known as the photosalient effect.
The first demonstration of the phenomenon of jumping crystals dates back to 1983, when a paper reported that crystals jump in response to a heat stimulus. This find challenged the conventional view of single crystals as rigid objects and was a proof of concept that they can produce mechanical work in response to external stimuli. Ever since, few cases of light and heat induced ’crystal jumping’ has been reported, but “they have never been explained before” says Pance Naumov, a chemist at the New York University Abu Dhabi.
“These materials could be applied in a variety of dynamic elements and actuating devices, including microfluidic lab-on-a-chip diagnostic devices, soft robotics and in printing technology,” he adds.
The NUS team, who reported the first incident of the effect induced by a photochemical reaction in the solid state, joined forces with Naumov’s group and Robert Dinnebier of the Max Planck institute of Solid State Research. Together, they used optical microscopy and powder X-ray diffraction to tap into the underlying reaction mechanism publishing their findings in Angewandte Chemie.
“Our results indicate that the photosalient effect is a two-step process” explains Naumov. “In the first step, a remarkable amount of strain is accumulated within the crystals as a result of a photochemical reaction whereby a portion of the crystal is converted into products.”
When a local instability is reached, either as a result of a defect in the structure or by applying an external pressure such as UV, a phase change is initiated that propagates rapidly along the crystal. “The stress that this change exerts causes blasting of the crystal into pieces, which fly off carrying most of the elastic energy as kinetic energy,” says Naumov.
“The study is an excellent demonstration of the untapped potential of organic solid state reactivity to material science,” says Leonard MacGillivray, professor of supramolecular chemistry at the University of Iowa, who was not involved in the study. “Although mechanical stress has been generally considered to build up in such materials, quantifying the observations has been a challenge.”
Light-driven actuators could one day replace conventional actuators powered by electricity or hydraulic power. “We believe that the path we set with the discovery of this phenomenon and the most recent demonstration of the general application of these materials set a proof to the concept of use of molecular materials for alternative energy conversion” says Naumov.
Now, the researchers are looking for other materials of this class where the crystals do not disintegrate by the end of the reaction.
Having a system where the conversion of photochemical energy into mechanical motion is reversible is important for future industrial applications, explains Naumov. “The system can be reverted back to the original state, ready for a new cycle, thus effectively operating as an energy transducer.”
“There is clearly great potential in such materials to perform work and exhibit motor-like functions. An important next step will likely be to further identify molecules that support such materials and gain control of the motion,” says MacGillivray.
doi:10.1038/nmiddleeast.2014.173
Stay connected: