Using AI to control energy for indoor agriculture
30 September 2024
Published online 28 February 2016
Bioengineered cows may be the answer in the fight against MERS-CoV.
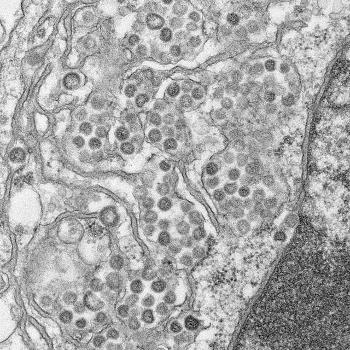
© Maureen Metcalfe/Azaibi Tamin
In recent years, researchers have made significant advances in understanding how MERS-CoV particles infect host cells, and while immunotherapy with neutralizing antibodies has been identified as a potential approach, as yet there is no effective treatment available.
However, Gabriel Defang of the Naval Medical Research Unit-3 in Cairo, along with colleagues and collaborators, had already reported two years earlier that they had isolated and characterized a new strain of the MERS-CoV, called Jordan-N3/2012, and used it to produce two different experimental vaccines against the virus2.
One of the vaccines, named SAB300, consists of entire Jordan strain viral particles, which have been killed, and leads to the production of antibodies against it. The other, SAB301, contains the spike glycoprotein from the Al-Hasa strain, which the virus uses to enter host cells.
The researchers used SAB301 to vaccinate two groups of cows whose immunoglobulin (Ig) genes – which produce antibodies – have been replaced with an artificial chromosome carrying the human Ig genes. When vaccinated, these cows develop a robust immune response, and produce fully human polyclonal antibodies against MERS-CoV.
DeFang and his colleagues then purified the antibodies from the cows’ blood, and tested them to evaluate their efficacy. They found that SAB301 effectively eliminated MERS-CoV from infected cells grown in Petri dishes, and that a single dose of it, administered to mice either 12 hours before or 24 and 48 hours after MERS-CoV infection, protected the animals from the virus.
The bioengineered cows used in the study produced between 150 – 600g of the antibodies per month, so the results, which have just been published in the journal Science Translational Medicine, suggest that this approach could offer a valuable new platform for the mass production of treatments for MERS-CoV and other emerging infectious diseases.
Kanakatte Raviprakash of the Naval Medical Research Center in Silver Spring, Maryland, a co-author of the study, says the next step is to file an application with the US Food and Drug Administration to start phase I studies in humans. “Availability depends upon many factors including the results of Phase II/III efficacy trials. We hope that if safety and efficacy is proven, then sufficient therapeutic would be available to those in need of treatment.”
MERS-CoV emerged in Saudi Arabia in 2012, and has been exported by human to other parts of the world. The World Health Organization has identified MERS-CoV as “a threat to global health,” and as of mid-November 2015, there have been 1,618 confirmed cases, and 579 deaths.
doi:10.1038/nmiddleeast.2016.20
Stay connected: